

Delivering patient centric clinical trials
Increasing recruitment & improving retention.
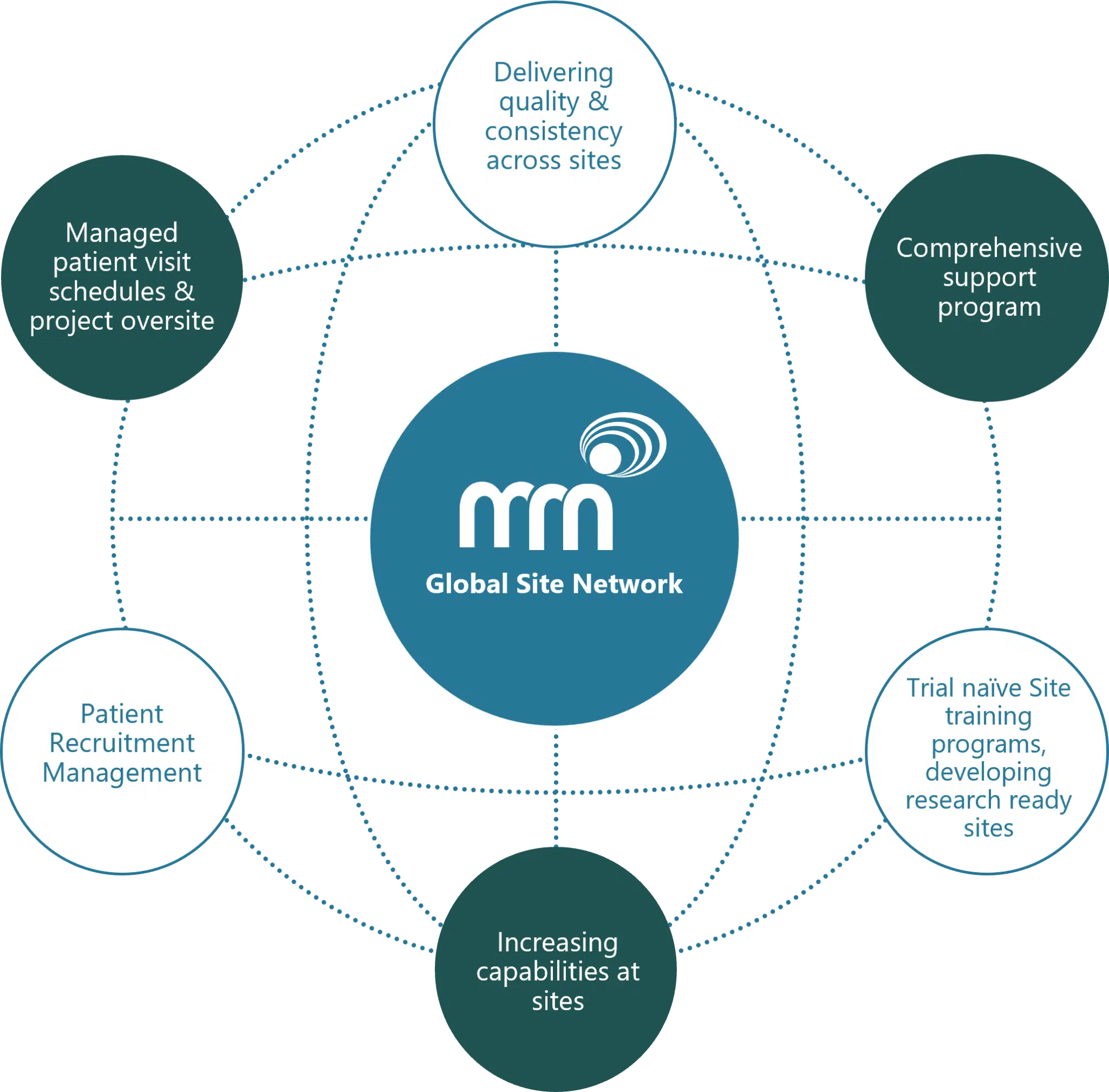
GLOBAL SITE NETWORK
Patient-centric delivery to keep patients at the heart of research, creating site resources and expanding site capabilities, join the clinical trial delivery experts, our mission.
At MRN we understand the complexities of today’s clinical trial environment and the burden this places on patients, clients and sites. Our services help ease these burdens, from community researchers through to investigator site professional support, accelerating patient recruitment and retention. We work to maximize the efficiency of clinical trials for drug developers, by improving the patient’s experience, no matter where their community is in the world.
Latest News

Supporting Research And Supporting Patients Through Community-Based Clinical Trials

Breaking Down Barriers: Improving Patient Access Through Home-Based Trials & Site Networks

Pushing Boundaries in Clinical Research: Reflections on DPharm 2024


ABOUT MRN DIAGNOSTICS

MRN Diagnostics provides a comprehensive toolbox of products and services to the diagnostics, biotechnology, and pharmaceutical industries for the research, development, and commercialization of RUO and In vitro diagnostic tests. We provide scientific expertise, critical biological materials, GMP manufacturing, analytical service testing and a secure biorepository to support all phases of diagnostic development. Some of this includes:
Prospective sample collections for cancer, infectious, cardiac, hepatitis and pulmonary diseases.
Archive of 250,000 remnant delinked clinical specimens from global collections
Analytical Services Division for high complexity CLIA testing, antigen, antibody and molecular testing
ISO13485:2016 Certified manufacturing facilities
FDA Registered Manufacturer
Raw bulk biomaterials (serum, plasma, WB) or processed into stabilized control matricies and finished products
Large inventory of validation and seroconversion panels for development
MEET THE EXECUTIVES
Gregory r. chiklis, phd..
Chief Executive Officer
Chief Scientific Officer
Dr. Chiklis has developed and commercialized thousands of diagnostic research products over his career., including In-vitro kit controls, calibrators, and multiple analytical services for assay validation. These are all designed to support diagnostic development, validation, manufacturing, commercialization, and quality control. Many of these products have been used to support test kit regulatory submissions and to quality control millions of test results annually.
Dr. Chiklis graduated from the University of Massachusetts, Amherst in 1986, with a B.S. in Biochemistry, and later from the University of Massachusetts Lowell with a Doctorate in Chemistry in 1992. He began his career at a start-up, Hemagen Diagnostics, where he developed in-vitro autoimmune and infectious disease assays. Next, he moved to BioClinical Partners, which eventually became ZeptoMetrix Corporation. There, as Vice President of Research and Development and eventually CEO, he developed and commercialized many more products including his patented NATtrol, noninfectious nucleic acid testing controls. Dr. Chiklis will use MRN's extensive biospecimen archive, prospective collection capabilities, research, and manufacturing facilities to continue to develop state-of-the-art diagnostics.
ROBERT O. MCKIE
Chief Financial Officer
Chief Operating Officer
Mr. McKie began his career as a Sales Representative for North American Biologicals in 1986. Continuing his healthcare focus in 1990 he became a Senior Sales Representative with Dianon Systems, Inc. He then moved on to Boston BioMedica, Inc. where from 1992 to 1994, he grew his responsibilities to become Director of Sales. In 1994, Mr. McKie co-founded BioClinical Partners, Inc. and served as Chief Operating Officer until 1999, when the business was acquired by IMPATH Inc. He was Vice President of Operations for the IMPATH Predictive Oncology™ division and was actively involved in the development and daily management of the company's GeneBank™ initiative and the IMPATH Clinical Trials Network™ (ICTN). Following his work with IMPATH, Mr. McKie provided consulting services on behalf of hospitals and life science companies. He successfully developed clinical services worldwide, providing protocol-directed and IEC-approved clinical studies that continue to deliver thousands of biospecimens in oncology and infectious diseases annually. Mr. McKie also holds a BS degree in Business from Nova University. At Medical Research Networx, Mr. McKie provides strategic direction for the company with a vision to become the largest and most respected supplier of biospecimens and ancillary products and services to the diagnostics industry.
COMPANY HISTORY
Mr. McKie founded Bioclinical Partners to provide a source of high quality biospecimens with accurate annotation, and a clear chain of custody.
Bioclinical Partners was acquired by IMPATH.
Mr. Morin joined Bioclinical Partners and later formed Bay State Biologicals to continue making high-quality biospecimens available to industry researchers.
Mr. McKie and Mr. Morin formed Medical Research Networx to provide a broad array of services and products to enable research and development of laboratory testing in the life science industry.
Dr. Gregory Chiklis joined the company as CEO/CSO and built a state of the art biorepository and a diagnostic research and manufacturing facility in Franklin, MA where the company now resides.
The COVID-19 Pandemic began, and MRN Diagnostics became one of the first points-of-care for COVID-19 testing, collecting, and researching.
Today, MRN Diagnostics offers multiple products and services for Research, Development, Validation, Manufacturing and commercialization of diagnostic assays.
Dr. Gregory R. Chiklis joined, building a manufacturing facility to manufacture and commercialize hundreds of diagnostic cancer products.

JOIN OUR TEAM
- Phlebotomists
- Laboratory Technicians
- Manufacturing/Assembly Technicians
- Summer Internships
- Office Assistants
- Data Entry
-Quality Control/Quality Assurance
-Materials Management
Keep up with us on LinkedIn and Indeed to stay updated on our open positions and opportunities.
STAY UPDATED ON MRNDx NEWS
Medical Research Networx

- © 2024
- Powered by A2Z Events

- Expo Hall Schedule
- Exhibitor Key Contacts
- Attendee Demographics
- Exhibitor Login
- DxPx Start-Up City
Medical Research Networx Diagnostics
- Booth: 4423
Medical Research Networx LLC (MRNdx). provides a comprehensive toolbox of services and products to the in-vitro diagnostics, biotechnology, and pharmaceutical industry for research, development manufacturing and commercialization of diagnostic tests. We provide expertise and critical materials to support the discovery, development and launch of immunoassay, molecular-genetic, anatomic pathology and hematology-based laboratory tests. MRN promotes complete transparency under signed CDA’s. MRN products and services include Human Biological materials obtained from within the US and around the globe including ZIKA, Dengue and Chikungunya virus; IRB approved De-Linked remnant specimens, fully consented clinical specimens, Bulk infectious and normal Serum and Plasma for Manufacturing of Controls, Calibrators, and Launch Panels for Marketing, Proficiency Panels, and Quality Control Panels and management of client specific prospective patient collections.
- Infectious Disease, Other
- Molecular Diagnostics
- Blood Banking
- Blood Collection
- OEM Components
- Clinical Research Services
- Research Products
- Biologicals
- Blood Products
- Diabetes Markers
- Stroke Markers
- Consulting Services
- Cancer Markers
- Cardiac Markers
- Tumor Markers
- Sun Jul, 24
- Mon Jul, 25
- Tue Jul, 26
- Wed Jul, 27
- Thu Jul, 28
- AACC Annual Scientific Meeting & Clinical Lab Expo Management
- SPARGO, Inc.
- Toll Free (800) 564-4220
- Phone: (703) 631-6200
- Fax (703) 563-2691
- Email: [email protected]

